INTRODUCTION:Previous clinical trials have shown that in situ vaccination using the combination of low-dose radiotherapy and intratumoral injection of CpG, a Toll-like receptor 9 (TLR9) agonist, can elicit T cell immune responses in patients with low grade lymphoma and regression at both the treated and non-treated distal tumor sites in patients with low-grade lymphoma (Brody et al., JCO 2010, Frank et al., Cancer Discovery 2018, Hammerich et al., Nat Med 2019). OX40 is a T cell activating co-receptor, belonging to the TNFR superfamily. Ligation of this receptor by an agonist anti-OX40 antibody leads to enhanced T cell activation of effector CD4 T cells while inhibiting Treg function. In preclinical models, addition of an anti-OX40 antibody to intratumoral CpG cured established tumors (Sagiv-Barfi I, et al. Sci Transl Med. 2018). Intratumoral injection of CpG up-regulated OX40 on CD4 T cells in the microenvironment enhancing the binding of agonistic anti-OX40 antibody that triggered a robust immune response. These results provided the rationale for a clinical trial of in situ vaccination (NCT03410901) using a human IgG1 agonistic anti-OX40 antibody (BMS-986178).
STUDY:Fourteen patients with low-grade lymphoma received low-dose (2Gy x 2) radiotherapy to a single tumor site followed by 5 weekly intratumoral injections of 2 mg CpG-ODN (SD-101, TriSalus Life Sciences) and 3.75 mg anti-OX40 antibody into the same site. 40mg of anti-OX40 was administered intravenously starting on day 2 and continuing every 4 weeks for a total of 6 infusions. Therapy was well tolerated with no dose-limiting toxicities. Response to therapy was assessed using the revised Lugano criteria (Cheson et al., JCO 2014) based on CT scans at 12, 24, 48, 72, and 96 weeks.
Fine needle aspirates (FNA) were performed at both the injected and a non-injected site outside the radiation field prior to, and at 1 and 6 weeks after initiation of treatment. Cells were analyzed by flow cytometry and droplet-based single-cell RNA sequencing to determine population dynamics, response to therapy as well as antibody binding and target occupancy by the anti-OX40 antibody.
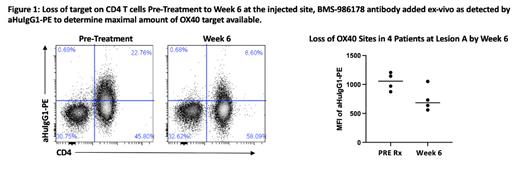
RESULTS:Of the 14 patients enrolled, 1 patient attained a partial response (PR), while 9 had stable disease (SD) and 4 had disease progression (PD) as their best overall response. Investigation into the binding of the treatment antibody and target occupancy by flow cytometry showed saturation of all the OX40 sites on CD4 T cells by week 6 at both the injected and non-injected tumors. However, further investigation also showed there was significantly less OX40 on the cell surface after treatment, suggesting a loss of the antibody target (Figure 1). This loss was reproduced by in vitro assays where activated blood CD4 T cells exhibited a loss of cell surface OX40 when cultured with BMS-986178. Additionally, Quigley et al ( Clin Cancer Res 2019) previously showed that an OX40 receptor occupancy of greater than 40% led to a loss of target in mice and in blood T cells of solid tumor patients. We further observed that the loss of cell surface OX40 occurred in all CD4 T subsets, including effector CD4 T cells and T Regulatory cells (Tregs). This result contrasted with our in vivo and in vitro findings in successful therapeutic mouse models where we did not observe this loss of cell surface OX40 on effector CD4 T cells after treatment with a mouse-specific anti-OX40 agonist antibody.
C ONCLUSION: Our observations suggest that treatment with an agonistic anti-OX40 antibody induced a loss of OX40 on the cell surface of effector CD4 T cells in the tumor microenvironment. Fewer molecules of OX40 receptor may have constrained the efficacy of subsequent anti-OX40 infusions and may explain why the clinical responses observed in this study were lower than observed in our past clinical trials of in situ vaccination.
Disclosures
Shree:Gilead: Other: Husband is working for Gilead. Levy:GigaGen: Consultancy; Abintus Bio: Consultancy; Kira Pharmaceuticals: Consultancy; Apexigen: Consultancy; Quadriga BioSciences: Consultancy; BeiGene: Consultancy; TeneoBio: Consultancy; Dragonfly: Consultancy; Nurix: Consultancy; Immunocore: Consultancy; Walking Fish Therapeutics: Consultancy; Viracta Therapeutics: Consultancy; BiolineRx: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal